Background
The combination of a proteasome inhibitor (PI) an immunomodulatory agent (IMiD), an anti-CD38 monoclonal antibody (anti-CD38MoAb) and dexamethasone, i.e. quadruplet therapy (QUAD) leads to highest rate of complete responses (CR) and prolonged progression-free survival (PFS) in newly diagnosed multiple myeloma (NDMM) in the context of autologous stem cell transplantation (ASCT). While there are treatment failures reported after QUADs and they may be exquisitely challenging to treat the published literature in this setting is scarce.
Methods
We included all patients treated with QUADs between January 2016 and May 2023 with intention to proceed with ASCT to identify treatment failures and report outcomes. Treatment failures were classified into primary failure (PF) defined as lack of objective response after at least 4 QUAD cycles or progression during or within 60 days of completing QUAD therapy; early progression (EP), defined as disease progression 2-12 months of completing QUAD therapy, and late progression (LP) defined as disease progression > 12 months from completing QUAD therapy. For each of these groups we analyzed disease features, treatment refractoriness (as patients in EP and LP may have varying refractoriness depending on maintenance therapy received), response to subsequent therapy and outcomes- Overall response rates(ORR), Progression free survival(PFS) and Overall Survival(OS).
Results
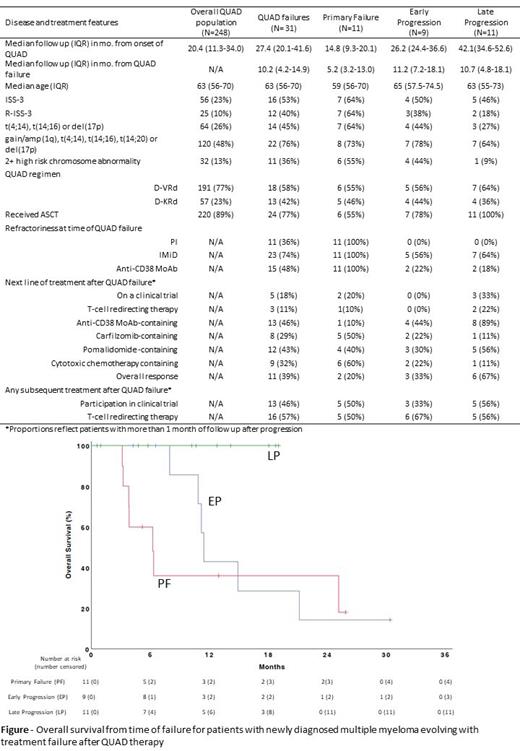
We identified 248 sequential patients treated with QUADs, including 191 (77%) treated with daratumumab, bortezomib, lenalidomide and dexamethasone (D-VRd), and 57 (23%) treated with daratumumab, carfilzomib, lenalidomide and dexamethasone (D-KRd). Median follow up for all patients is 20.4 months (IQR 11.3-34.0) from onset of QUAD therapy. We found 11 patients with PF, 9 with EP and 11 with LP (Table) for a total of 31 treatment failures. Patients with QUAD failures were more likely to have ISS-3, R-ISS3 and 2+ high-risk chromosome abnormalities than the overall QUAD population. Median follow up from QUAD failure is 5.2 months for PF, 11.2 months for EP and 10.7 months for LP. All 31 patients with QUAD failure received subsequent therapy and 28 patients have > 1 month follow up enable assessment of response to subsequent therapy (Table). Cytotoxic chemotherapy was frequently used in patients with PF (60%) whereas anti-CD38 monoclonal Ab containing regimen was commonly used with increasing time from initial QUAD therapy reaching 89% in patients with LP. ORR to next line of therapy was 20% for PF, 33% for EP and 67% for LP. Of the 28 patients, 13(46%) participated in subsequent treatment clinical trial and 16(57%) received subsequent T-cell redirecting therapy either bispecific antibody or CAR-T cell therapy . Median PFS on next line of therapy was 2.4 months for PF, 3.7 months for EP and 7.4 months for LP. Median OS from QUAD failure was only 6.3 months for PF and 11.5 months for EP. No deaths (0/11) observed in LP group (Figure).
Conclusions
Multiple myeloma patients with delayed progression (>12 months) from end of QUADs have reasonable response to subsequent therapy. Despite access to experimental therapies including T-cell redirected therapies, patients with earlier failure of QUAD therapy (0-12 months) have poor response rates to subsequent treatments and significantly shorter survival. These findings highlight the urgent need for better predictive models to identify patients with QUAD failure and preemptively deploy treatments with novel mechanisms of action.
Disclosures
Bal:Fate Therapeutics: Research Funding; Adaptive Biotechnology: Consultancy; Beigene: Research Funding; MJH Lifesciences: Other: Educational content development ; Amyloid Foundation: Research Funding; AbbVie: Consultancy; Astrazeneca: Consultancy; Janssen: Consultancy; Bristol Myers Squibb: Consultancy. Costa:BMS: Consultancy, Honoraria, Research Funding; Amgen: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria, Research Funding; Pfizer: Consultancy, Honoraria; AbbVie: Honoraria, Research Funding; Adaptive biotechnologies: Consultancy, Honoraria; Genentech: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal